Answer:
86.2 g/mol
Step-by-step explanation:
Before you can find the molar mass, you first need to calculate the number of moles of the gas. To find this value, you need to use the Ideal Gas Law:
PV = nRT
In this equation,
-----> P = pressure (mmHg)
-----> V = volume (L)
-----> n = moles
-----> R = Ideal Gas constant (62.36 L*mmHg/mol*K)
-----> T = temperature (K)
After you convert the volume from mL to L and the temperature from Celsius to Kelvin, you can use the equation to find the moles.
P = 760 mmHg R = 62.36 L*mmHg/mol*K
V = 250 mL / 1,000 = 0.250 L T = 20 °C + 273.15 = 293.15 K
n = ? moles
PV = nRT
(760 mmHg)(0.250 L) = n(62.36 L*mmHg/mol*K)(293.15 K)
190 = n(18280.834)
0.0104 = n
The molar mass represents the mass (g) of the gas per every 1 mole. Since you have been given a mass and mole value, you can set up a proportion to determine the molar mass.
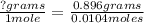 <----- Proportion
<----- Proportion
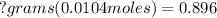 <----- Cross-multiply
<----- Cross-multiply
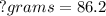 <----- Divide both sides by 0.0104
<----- Divide both sides by 0.0104