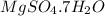Answer:
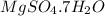
Step-by-step explanation:
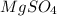 is an ionic compound because aluminium element is a metal and hydrogen element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
is an ionic compound because aluminium element is a metal and hydrogen element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
The nomenclature of ionic compounds is given by:
1. Positive is written first.
2. The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written in case of
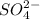 is '-ate'.
is '-ate'.
Hence, the name of
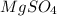 is magnesium sulphate.
is magnesium sulphate.
The water of crystallization is written as hydrate with the number of water molecules. As the name is heptahydrate, that means 7 water molecules are present.
Therefore, the correct answer is