Answer:
Na₂CO₃.2H₂O
Step-by-step explanation:
For the hydrated compound, let us denote is by Na₂CO₃.xH₂O
The unknown is the value of x which is the amount of water of crystallisation.
Given values:
Starting mass of hydrate i.e Na₂CO₃.xH₂O = 4.31g
Mass after heating (Na₂CO₃) = 3.22g
Mass of the water of crystallisation = (4.31-3.22)g = 1.09g
To determine the integer x, we find the number of moles of the anhydrous Na₂CO₃ and that of the water of crystallisation:
Number of moles =
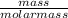
Molar mass of Na₂CO₃ =[(23x2) + 12 + (16x3)] = 106gmol⁻¹
Molar mass of H₂O = [(1x2) + (16)] = 18gmol⁻¹
Number of moles of Na₂CO₃ =
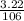 = 0.03mole
= 0.03mole
Number of moles of H₂O =
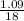 = 0.06mole
= 0.06mole
From the obtained number of moles:
Na₂CO₃ H₂O
0.03 0.06
Simplest
Ratio 0.03/0.03 0.03/0.06
1 2
Therefore, x = 2