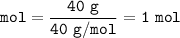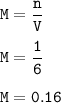Molarity of solution = 1.6 M
Further explanation
Given
40 g NaOH
6 L solution
Required
Steps to solve the problem of molarity
Solution
No additional information about the question.
If you want to make the solution above, then we just need to put the existing NaOH (40 g) into 6 L of water, then do the stirring (in a warm temperature above the hot plate will speed up the NaOH dissolving process)
But if you want to know the molarity of a solution, then
- 1. we calculate the moles of NaOH
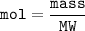
MW(molecular weight) of NaOH=
Ar Na+ Ar O + Ar H
23 + 16 + 1 = 40 g/mol
so mol NaOH :