Answer: A. an exothermic reaction
Step-by-step explanation:
A. Exothermic reactions are defined as the reactions in which energy of the product is lesser than the energy of the reactants. The total energy is released in the form of heat. The temperature of the vessel rises.
B. Decomposition is a type of chemical reaction in which one reactant gives two or more than two products.
Example:
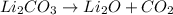
C. Single displacement reaction is a chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
Example:
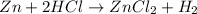
D. Endothermic reactions are defined as the reactions in which energy of the product is greater than the energy of the reactants. The total energy is absorbed in the form of heat.The temperature of the vessel drops.