The given question is incomplete, here is a complete question.
The reaction below shows a system in equilibrium.
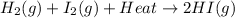
How would a decrease in temperature affect this reaction?
A. The rate of formation of the gases would increase.
B. The equilibrium of the reaction would shift to the left.
C. The equilibrium would shift to cause the gases to sublime into solids.
D. The chemicals on the left would quickly form the chemical on the right.
Answer : The correct option is, (B) The equilibrium of the reaction would shift to the left.
Explanation :
The given reaction is endothermic reaction.
For an endothermic reaction, heat is getting absorbed during a chemical reaction and is written on the reactant side.
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle. This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
As, heat is getting absorbed during a chemical reaction. This means that temperature is getting increased on the reactant side.
If the temperature in the equilibrium is decreased, the equilibrium will shift in the direction where, temperature is getting increased. Thus, the reaction will shift in left direction that is towards the reactants.
Hence, the correct option is, (B) The equilibrium of the reaction would shift to the left.