Answer: If a substance is the limiting reactant, then it will be completely used by the time the reaction completes because it is the reactant that reacts completely and the reaction stops after it is completely used up.
Step-by-step explanation:
Limiting reagent is a reagent as it limits the formation of product and Excess reagent is the reagent which is preset in excess.
For example: if 0.75 moles of HCl and 1.5 moles of magnesium are reacted together.
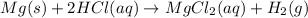
According to stoichiometry:
As 2 mole of
 reacts with 1 mole of
reacts with 1 mole of
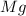
0.75 moles of
 will react with =
will react with =
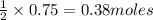 of
of
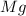
 is a limiting reagent as it limits the formation of product and Mg is an excess reagent as (1.5-0.38)=1.12 moles of magnesium are left unreacted.
is a limiting reagent as it limits the formation of product and Mg is an excess reagent as (1.5-0.38)=1.12 moles of magnesium are left unreacted.