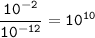The ratio of H⁺ ions to OH⁻ ions at a pH = 2 is 10¹⁰
Further explanation
Given
ph = 2
Required
The concentration of H⁺ and OH⁻ ions
Solution
- The concentration of H⁺ ions
pH=-log[H⁺]
2=-log[H⁺]
[H⁺]=10⁻²
- The concentration of OH⁻ ions
pH+pOH=14
pOH=14-2
pOH=12
pOH=-log[OH⁻]
12=-log[OH⁻]
[OH⁻]=10⁻¹²
- The ratio of H⁺ ions to OH⁻ ions at a pH = 2