Answer:
Option A, occurs at a constant rate
Step-by-step explanation:
It becomes easy to determine the age of any radioactive substance when the rate of decay is constant and its starting and presents mass is known.
For example - if we know that the mass of a radioactive substance is 100 Kg few years back , but its present mass is 10 Kg. Then if we know the value of rate of decay, we can easily determine the age of substance based on the logarithmic equation as given below
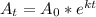
Where
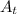 is the mass of radioactive substance at time t
is the mass of radioactive substance at time t
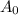 is the mass of radioactive substance at time
is the mass of radioactive substance at time
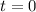
t is the time in years and
k is the decay constant.
Hence, option A is correct.