Answer:
The volume of the car tire is 53.93 Liters.
Step-by-step explanation:
Pressure of the gas in car tire =
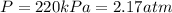
(1 kPa = 0.009869 atm)
Temperature of the gas in tire =T = 297 K
Moles of air in the tire = n = 4.8 moles
Volume of the gas in the tire= V
Using an ideal gas equation:


V = 53.93 L
The volume of the car tire is 53.93 Liters.