Answer: The number of molecules in given amount of sulfuric acid is

Step-by-step explanation:
According to the mole concept:
1 mole of a compound contains
 number of molecules.
number of molecules.
We are given:
Moles of sulfuric acid = 2.25 mole
So, the number of molecules that will be present in 2.25 moles of sulfuric acid will be
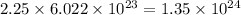 number of atoms.
number of atoms.
Hence, the number of molecules in given amount of sulfuric acid is
