Answer:
The minimum volume of the container is 0.0649 cubic meters, which is the same as 64.9 liters.
Step-by-step explanation:
Assume that ethane behaves as an ideal gas under these conditions.
By the ideal gas law,
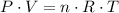 ,
,
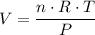 .
.
where
 is the pressure of the gas,
is the pressure of the gas,
 is the volume of the gas,
is the volume of the gas,
 is the number of moles of particles in this gas,
is the number of moles of particles in this gas,
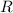 is the ideal gas constant, and
is the ideal gas constant, and
 is the absolute temperature of the gas (in degrees Kelvins.)
is the absolute temperature of the gas (in degrees Kelvins.)
The numerical value of
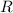 will be
will be
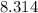 if
if
 ,
,
 , and
, and
 are in SI units. Convert these values to SI units:
are in SI units. Convert these values to SI units:
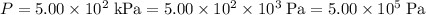 ;
;
 shall be in cubic meters,
shall be in cubic meters,
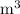 ;
;
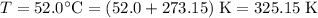 .
.
Apply the ideal gas law:
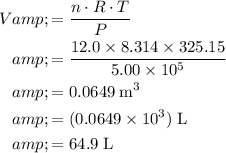 .
.