Answer:
d) 1/1840 of hydrogen atom mass is the relative electron mass.
Step-by-step explanation:
The hydrogen gas is the lightest element and its atomic mass is
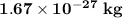 , same as the mass of proton because it has no neutron and contains one proton and one electron in its atom. But it loses an electron to gain stable electronic configuration leaving only a proton in its atomic structure.
, same as the mass of proton because it has no neutron and contains one proton and one electron in its atom. But it loses an electron to gain stable electronic configuration leaving only a proton in its atomic structure.
We know, Mass of an electron =
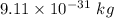
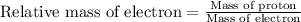
Relative mass of electron =
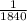 the mass of proton
the mass of proton
Relative mass of electron =
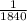 the mass of hydrogen atom.
the mass of hydrogen atom.