Answer:
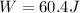
Work done is 60.4 Joules (J)
Step-by-step explanation:
Work done 'W' is given by:
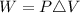
Where:
ΔV is the change in Volume.
P is the pressure.
Change in Volume=Final Volume-Initial Volume
Initial Volume= 127 mL
Final Volume= 654 mL
ΔV=654-127 (mL)
ΔV=527 mL
ΔV=0.527 L
Pressure Conversion:
1 atm=760 torr
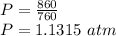
Now,
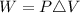
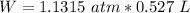
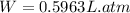
In joule (J): (Conversion 1 atm. L=101.325 J)
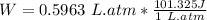
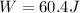
Work done is 60.4 Joules (J)