Answer : The formula unit of compound will be,
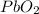
Explanation :
As we know that one atom of oxygen contains (2-) charge and one atom of lead contains (4+) charge. So, to balance the of charges of lead, the 2 oxygen atoms combine with the 1 lead atom. Thus, the compound formed will be,
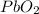 .
.
That means, when
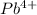 combine with oxygen that is,
combine with oxygen that is,
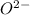 , it gives
, it gives
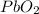 by the criss-cross method.
by the criss-cross method.
Criss cross method : It is a method of determining the chemical formula.
The formula unit is a shown below.