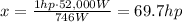(a)
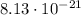
The magnitude of the charge of one electron is
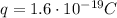
Here the total amount of charge that passed through the battery pack is
Q = 1300 C
So this total charge is given by
Q = Nq
where
N is the number of electrons that has moved through the battery
Solving for N,
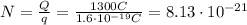
(b)
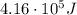
First, we can find the current through the battery, which is given by the ratio between the total charge (Q = 1300 C) and the time interval (t = 8.0 s):
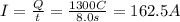
Now we can find the power, which is given by:
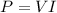
where
V = 320 V is the voltage
I = 162.5 A is the current
Subsituting,
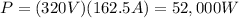
And now we can find the total energy transferred, which is the product between the power and the time:
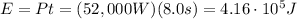
(c) 69.7 hp
Now we have to convert the power from Watt to horsepower.
We know that
1 hp = 746 W
So we can set up the following proportion:
1 hp : 746 W = x : 52,000 W
And by solving for x, we find the power in horsepower: