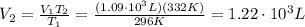Answer:
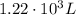
Step-by-step explanation:
We can solve the problem by using Charle's law, which states that for a gas kept at constant pressure, the volume of the gas is directly proportional to its temperature:
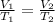
where here we have
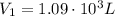 is the initial volume
is the initial volume
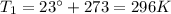 is the initial temperature
is the initial temperature
 is the final volume
is the final volume
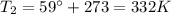 is the final temperature
is the final temperature
Solving for V2, we find