Answer:
The correct answer is:
'He did not multiply the chlorine and oxygen atoms by the coefficient 4'.
Step-by-step explanation:
Number of atoms in
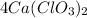
The 4 in front of molecular formula of calcium chlorate is its coefficient . it tells us about the molecules or moles of compound.
Number of calcium in 1 molecule of calcium chlorate = 1
Calcium atoms in 4 molecule of calcium chlorate = 1 × 4 = 4
Number of calcium in 1 molecule of calcium chlorate = 2
Chlorine atoms in 4 molecule of calcium chlorate = 2 × 4 = 8
Number of oxygen in 1 molecule of calcium chlorate = 3 × 2 = 6
Oxygen atoms in 4 molecule of calcium chlorate = 6 × 4 = 24
So, number of atoms of different elements calculated by Carl was wrong as he didn't multiplied the coefficient 4 with chlorine and oxygen atoms.