Answer:
There are approximately d. 0.595 mol of ZnCl₂ in 3.58×10²³ formula units.
Step-by-step explanation:
Consider the Avogadro's Constant
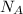 or equivalently,
or equivalently,
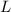 :
:
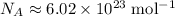 .
.
In other words, there are
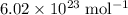 constituent particles (Wikipedia) in each mole of a substance. In this case,
constituent particles (Wikipedia) in each mole of a substance. In this case,
- Zinc chloride
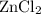 is the substance, and
is the substance, and
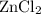 formula units are the constituents.
formula units are the constituents.
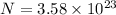 .
.
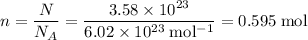 .
.