Answer: The electronic configuration is given below.
Step-by-step explanation:
Electronic configuration tells us about the number of electrons that are present in an atom. It also determines the atomic number of an element.
Boron is the 5th element of the periodic table having 5 electrons.
The electronic configuration for the given element =
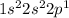
This element has 3 valence electrons.
Thus, the electronic configuration is given above.