Answer:
Atomic mass
Step-by-step explanation:
The weighted average of the masses of the various isotopes of an element makes the atomic mass. This is why most atomic mass values usually have decimals.
In order to calculate the atomic mass of an element using the weighted average masses, we use the expression below:
Atomic mass = ∑
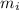
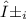
Where
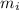 is the mass of isotope i
is the mass of isotope i
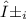 is the abundance of isotope i
is the abundance of isotope i
The abundance or geonormal abundance is the proportion by which each of the isotope occurs in nature.