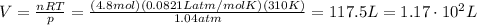Answer:
1.17 x 10^2 L
Step-by-step explanation:
We can find the volume of the gas by using the ideal gas law:
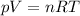
where we have:
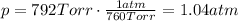 is the pressure
is the pressure
V is the volume
n = 4.8 mol is the number of moles
R = 0.0821 L · atm/mol · K is the ideal gas constant
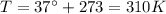 is the temperature
is the temperature
Solving the equation for V, we find the volume