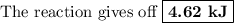Answer:
Step-by-step explanation:
There are two heat transfers to consider:
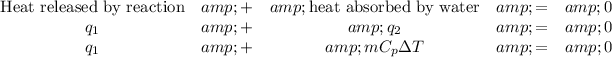
Calculate q₂
m = 120.0 g
C = 4.184 J·°C⁻¹g⁻¹
T₂ = 29.20 °C
T₁ = 20.00 °C
ΔT = T₂ - T₁ =(29.20 – 20.00) °C =9.20 °C
q₂ = 120.0 g × 4.184 J·°C⁻¹g⁻¹ × 9.20 °C = 4620 J = 4.62 kJ
Calculate q₁
q₁ + 4.62 kJ = 0
q₁ = -4.62 kJ
The negative sign shows that heat is given off.