Answer:
There are
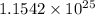 molecules of water are in 345 grams.
molecules of water are in 345 grams.
Step-by-step explanation:
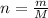
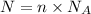
Where:
m = mass of compound
M = Molar mass of compound
N = Number of particles / atoms/ molecules
n = Number of moles
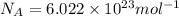 = Avogadro's number
= Avogadro's number
Mass of water = m = 345 g
Molar mas of water = M = 18 g/mol
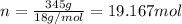
Number of molecules of water = N
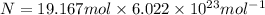

There are
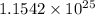 molecules of water are in 345 grams.
molecules of water are in 345 grams.