Answer:
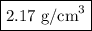
Step-by-step explanation:
1. Ions per unit cell
(a) Chloride
8 corners + 6 faces
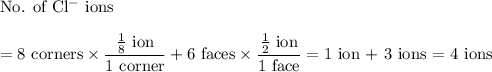
(b) Chloride
12 edges + 1 centre
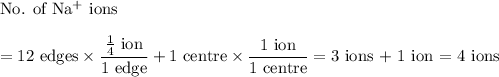
There are four formula units of NaCl in a unit cell.
2. Mass of unit cell
m = 4 × NaCl = 4 × 58.44 u = 233.76 u
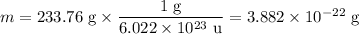
3. Volume of unit cell
(a) Edge length
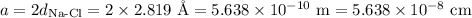
(b) Volume
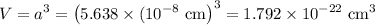
4. Density
