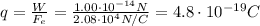Answer:
Step-by-step explanation:
For a drop in equilibrium, the weight is equal to the electric force (in magnitude):
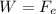
where here we have
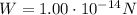 is the weight of the drop
is the weight of the drop
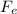 is the magnitude of the electric force, which can be rewritten as
is the magnitude of the electric force, which can be rewritten as
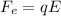
where
q is the charge of the oil drop
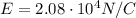 is the magnitude of the electric field
is the magnitude of the electric field
Substituting into the equation and solving for q, we find the charge of the oil drop: