Answer:
1)
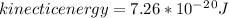
2)
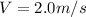
3)
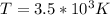
4) The Molecules do not burn because of the presences of hydrogen bond in place
Step-by-step explanation:
From the question we are told that
latent heat of vaporization for water at room temperature is 2430 J/g.
1)Generally in determining the molar mass of water evaporated we have that
-One mole (6.02 x 10. 23 molecules)
-Molar mass of water is 18.02 g/mol
Mathematically the mass of water is give as
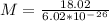

Therefore
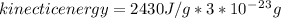
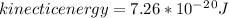
b)Generally the evaporation speed V is given as
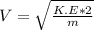
Mathematically derived from the equation
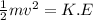
To Give
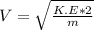
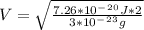
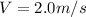
c)Generally the equation for velocity
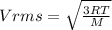
Therefore
Effective temperature T is given by
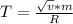
where
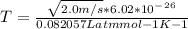
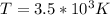
4) The Molecules do not burn because of the presences of hydrogen bond in place