Answer:
211.9 J
Step-by-step explanation:
The molecules of water release heat during the transition of water vapor to liquid water, but the temperature of the water does not change with it.
The amount of heat released can be represented by the formula:
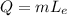
where
 = heat energy,
= heat energy,
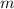 = mass of water and
= mass of water and
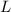 = latent heat of evaporation.
= latent heat of evaporation.
The latent heat of evaporation for water is
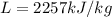 and the mass of the water is
and the mass of the water is
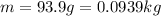 .
.
The amount of heat released in this process is:
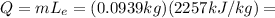 211.9 J
211.9 J