Answer:
B) The enthalpy of combustion = 3,084,840 J
Step-by-step explanation:
Given:
Moles of butane = 0.02
Mass of water, m = 328 g
Initial temperature T1 = 298 K
Final temperature T2 = 343 K
Specific heat of water, c = 4.18 J/g-K
To determine:
Enthalpy of combustion
Step-by-step explanation:
Heat lost during combustion of butane = heat gained by water
Heat gained (q) by water is given as:
q = mc\Delta T = mc(T2-T1)
substituting for m, c, T2 and
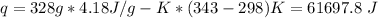 T1
T1
