Answer:
By adding baking soda
Step-by-step explanation:
The best way to reduce the acidity of the spill would be to add some amount of baking soda to the spill and then clean it up with a towel or any other appropriate item.
Baking soda is made up of sodium bicarbonate and has the capacity to react with acid to produce a salt and carbonic acid. The carbonic acid readily decomposes to produce carbon dioxide and water. For example, sodium bicarbonate reacts with hydrochloric acid according to the equation below:

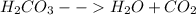
One would expect the addition of a base to the spilled acid to work in a way as to neutralize the acid but bases are also corrosive and only a stoichiometrically balanced quantity would completely neutralize the acid. Thus, the quantity might not easily be determinable and one can end up doing more harm than good.
Hence, the best option is to add baking soda to the spilled acid to reduce its acidity before cleaning it off.