Answer: a.
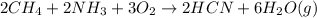
b.
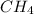 is the limiting.
is the limiting.
c. 13.5 g of hydrogen cyanide will be formed
Step-by-step explanation:
To calculate the moles :
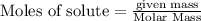
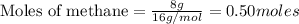
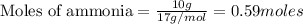
The balanced reaction will be :
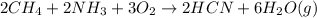
According to stoichiometry :
2 moles of
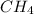 require = 2 moles of
require = 2 moles of
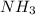
Thus 0.5 moles of
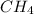 will require=
will require=
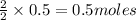 of
of
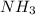
Thus
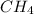 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
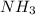 is the excess reagent as it is present in more amount than required.
is the excess reagent as it is present in more amount than required.
As 2 moles of
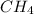 give = 2 moles of
give = 2 moles of
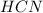
Thus 0.5 moles of
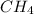 give =
give =
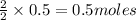 of
of
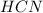
Mass of
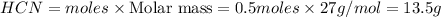
Thus 13.5 g of
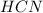 will be produced from the given masses of both reactants.
will be produced from the given masses of both reactants.