Step-by-step explanation:
Nuclear reactions are defined as the reaction in which there occurs change in the nucleus of an atom due to change in number of protons or neutrons of an atom.
For example,
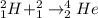 is a nuclear reaction.
is a nuclear reaction.
Hence, identity of the element changes in a nuclear reaction. Also, nuclear reaction can occur at varied rates as they are not affected by temperature, pressure or catalyst.
Whereas a chemical reaction is defined as the reaction where there occurs exchange of electrons between the combining atoms.
For example,
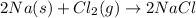 is a chemical change.
is a chemical change.
Thus, we can conclude that nuclear reactions are different from chemical reactions as follows.
- In nuclear reactions, the identities of the elements change.
- In nuclear reactions, the reactions happen at a varied rate.