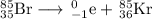Answer:
Step-by-step explanation:
Your nuclear equation is
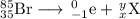
The main point to remember in balancing nuclear equations is that
- the sum of the superscripts and must be the same on each side of the equation.
- the sum of the subscripts must be the same on each side of the equation.
Then
85 = 0 + y, so y = 85 - 0 = 0
35 = -1 + x, so x = 35 + 1 = 36
The nucleus with atomic number 36 and atomic mass 85 is krypton-85.
The nuclear equation becomes