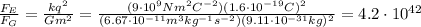Answer:
D) 10^42
Step-by-step explanation:
The electrostatic force between two electrons is given by
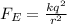
where
k is the Coulomb's constant
q is the electron charge
r is the separation between the electrons
The gravitational force between two electrons is
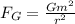
where
G is the gravitational constant
m is the electron mass
r is the separation between the electrons
So the ratio between the two forces is