Answer:
22g of He
Step-by-step explanation:
Volume of gas at STP is given as :
Volume occupied(dm³) = number of moles x 22.4dm³mol⁻¹
In order to solve this given problem, we would first find the number of moles from the different masses of atoms and molecules given.
Number of moles =
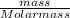
Now, let us obtain the molar mass of the given atoms and elements:
For Ne; molar mass is the atomic mass = 20gmol⁻¹
He = 4gmol⁻¹
O₂ = (16 x2)gmol⁻¹ = 32gmol⁻¹
Cl₂ = (35.5x2)gmol⁻¹ = 71gmol⁻¹
Number of moles of Ne =
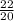 = 1.1mol
= 1.1mol
Volume occupied by Ne at STP = 1.1 x 22.4 = 24.64dm³
Number of moles of He =
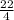 = 5.5mol
= 5.5mol
Volume occupied by He at STP = 5.5 x 22.4 = 123.2dm³
Number of moles of O₂ =
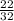 = 0.688mol
= 0.688mol
Volume occupied by O₂ at STP = 0.688 x 22.4 = 15.4mol
Number of moles of Cl₂ =
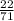 = 0.3099mol
= 0.3099mol
Volume occupied by Cl₂ at STP = 0.3099 x 22.4 = 6.94dm³