Answer:
2.83848 grams of
 are produced
are produced
Step-by-step explanation:
Assuming that the reaccions continues until the total consumption of the reagents, it is possible to establish:
1 mol Al = 1 mol
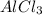
2 mol
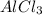 = 3 mol
= 3 mol

Then, by looking at the periodic table, it is possible to know the molar wheigt of the compounds:
1 mol Al = 29.9815 gr
1 mol
 = 2 gr
= 2 gr
1 mol
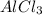 = 136.3405 gr
= 136.3405 gr
Reeplacing the moles with the respectly wheights of compounds:
29.9815 gr Al = 136.3405 gr
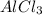
X gr Al = 129 gr
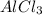
So, 129 grams of
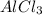 equals to 28.3673 grams of Al.
equals to 28.3673 grams of Al.
Then, following the same line of thought:
29.963 gr Al (2 moles) = 6 gr
 (3 moles)
(3 moles)
28.3673 gr Al = Y gr

So, 28.3673 grams of Al equals to 2.8384 grams of
 .
.