Answer:
During an alpha decay
Step-by-step explanation:
An alpha particle is a particle consisting of 2 protons and 2 neutrons - basically it is equivalent to a nucleus of helium.
Alpha particles are emitted during alpha decays, which are one of the three types of radioactive decays (the other two being beta decay and gamma decay) in which an unstable nucleus decays emitting an alpha particle:
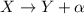
In the process, the original nucleus X loses 2 protons and 2 neutrons, so:
- its atomic number decreases by 2 units: Z --> Z-2
- its mass number decreases by 4 units: A --> A-4