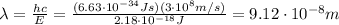Answer:
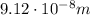
Step-by-step explanation:
The energy needed to ionize a hydrogen atom in the ground state is:
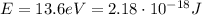
The energy of the photon is related to the wavelength by

where
h is the Planck constant
c is the speed of light
 is the wavelength
is the wavelength
Solving the formula for the wavelength, we find