Answer:
OB) 1.0 atm
Step-by-step explanation:
we have a mixture of hydrogen, nitrogen and methane and we have the following data
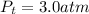
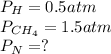
According to the law of Dalton the sum of the partial pressures of each gas is equal to the total pressure
Total pressure equation
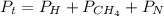
now we clear the partial pressure of nitrogen from the equation
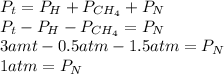
the partial pressure of nitrogen is 1 atm
the correct answer is OB) 1.0 atm