Answer:
Step-by-step explanation:
aCa + bCO₂ + cO₂ → dCaCO₃
Chemical reactions obey the law of conservation of matter. The law states that "matter is neither created nor destroyed in the course of a chemical reaction". From this, we can deduce that the total mass of the products and reactants are equal.
To balance a chemical reaction, we can do so by inspecting the atoms/compounds involved and putting the appropriate coefficients at the back of the reactants and products. For equations in which inspection might not easily work, we can use a mathematical approach.
By inspecting the reaction above, we can easily balance it
Using the mathematical approach:
The coefficients are a,b,c and d. They are needed to conserve the atoms. So:
For Conservation of Ca: a = d (i)
C: b = d (ii)
O: 2b + 2c = 3d (iii)
if we assume that a = 1, then d = 1
b = 1
from (iii): 2c = 3d -2b
2c = 3- (2 X 1)
2c = 1
c =
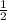
Therefore we have a = 1
c =
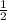
b = 1
d = 1
aCa + bCO₂ + cO₂ → dCaCO₃
Ca + CO₂ +
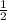 O₂ → CaCO₃
O₂ → CaCO₃