Answer:The value of the coefficients are:
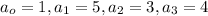
Step-by-step explanation:
Number written in front of the molecule or element in balanced chemical reaction is known as stoichiometric coefficient.
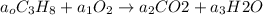
The balance chemical reaction will be given by:
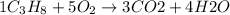
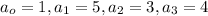
1 mole of octane reacts with 5 moles of oxygen gas to gives 3 moles of carbon dioxide and 4 moles of water as a product.