Answer:
0.139 moles of C3H8 must be reacted to form exactly 10.0 g of H2O
Step-by-step explanation:
The rule of three or is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them. That is, what is intended with it is to find the fourth term of a proportion knowing the other three. Remember that proportionality is a constant relationship or ratio between different magnitudes.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other (or when one magnitude decreases, so does the other) , the direct rule of three must be applied. To solve a direct rule of three, the following formula must be followed:
a ⇒ b
c ⇒ x
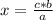
It is possible to use the reaction stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction) and the rule of three to determinate the moles of C₃H₈ that must be reacted to form exactly 10.0 g of H₂O. But first you must know the amount of moles that represent the 10 g of H₂O.
You know that:
Then, the mass of H₂O is 2*1 g/mol + 16 g/mol= 18 g/mol
Then it is possible to apply a rule of three: if 1 mole of H₂O contains 18 grams, how many moles will contain 10 grams?
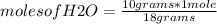
moles of H₂O=0.556
Then, to determine the moles of C₃H₈ that must react to form exactly 10.0 g of H₂O it is possible to use a rule of three, as previously mentioned: if by stoichiometry 4 moles of H₂O are formed from 1 moles of C₃H₈, when are formed 0.55 moles of H₂O How many moles of C₃H₈ will be needed?
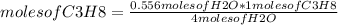
moles of C₃H₈= 0.139
Finally, 0.139 moles of C3H8 must be reacted to form exactly 10.0 g of H2O