Answer:
Molarity of NaOH = 0.212M, Molarity of HCl = 0.436M
Step-by-step explanation:
Before answering the question, it is crucial to write out the chemical equation between HCl and NaOH. This is given below as;
HCl + NaOH -> NaCl + H2O
HCl is the Acid and NaOH is the base.
From the reaction we can tell that 1 mole of HCl reacts with 1 mole of NaOH.
We use the acid base relationship in calculating unknown concentration;
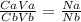
The question stated the following;
Volume of NaOH (Vb) = 25.0ml
Concentration of NaOH (Cb) = 0.212M
Volume of HCl (Va) = 13.6ml
Concentration of HCl (Ca) = ?
From the equation above;
Na = 1
Nb = 1
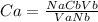
Cb = (1 * 0.212 * 25) / (13.6 * 1)
Cb = 0.436 M