Step-by-step explanation:
Chemical formula of a compound tells us about the number of atoms of elements that are present in a compound.
For the given options:
1. Nitric acid
This is an acid in which one hydrogen, 1 nitrogen and 3 oxygen atoms are present. The chemical formula for this compound is
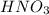
2. Sulfurous acid
This is an acid in which two hydrogen, 1 sulfur and 3 oxygen atoms are present. The chemical formula for this compound is
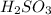
3. Hypochlorous acid
This is an acid in which one hydrogen, 1 chlorine and 1 oxygen atoms are present. The chemical formula for this compound is
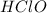
4. Ammonia
This is an acid in which three hydrogen and 1 nitrogen atoms are present. The chemical formula for this compound is

5. Sulfuric acid
This is an acid in which one hydrogen, 1 sulfur and 4 oxygen atoms are present. The chemical formula for this compound is
