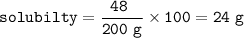The solubility of the salt. : 24 g/100 g solvent(water)
Further explanation
Given
48 gram of a salt in 200 gram of water at 35°C
Required
The solubility of the salt.
Solution
Solubility : the amount of a substance that can be dissolved in a solvent
Can be formulated(for 100 g solution)⇒grams solute per 100 g of solvent(100 ml water)
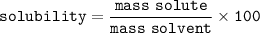
Input the value :