Answer:
Step-by-step explanation:
Pair 2.50g of O₂ and 2.50g of N₂
The atoms sample with the largest number of moles since the masses are the same would be the one with lowest molar mass according the the equation below:
Number of moles =
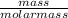
Atomic mass of O = 16g and N = 14g
Molar mass of O₂ = 16 x 2 = 32gmol⁻¹
Molar mass of N₂ = 14 x 2 = 28gmol⁻¹
Number of moles of O₂ =
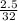 = 0.078mole
= 0.078mole
Number of moles of N₂ =
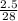 = 0.089mole
= 0.089mole
We see that N₂ has the largest number of moles