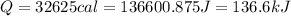Answer: 136.6 kJ
Step-by-step explanation:
The amount of heat
 absorbed in the temperature variation of a material is:
absorbed in the temperature variation of a material is:
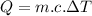 (1)
(1)
Where:
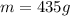 is the mass of water
is the mass of water
 is the specific heat of the element. In the case of water
is the specific heat of the element. In the case of water
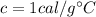
 is the variation in temperature, which in this case is
is the variation in temperature, which in this case is
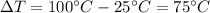
(The boiling point of water at the pressure of 1 atm is
 )
)
Rewriting equation (1) with the known values:
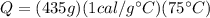
(2)
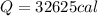 (3)
(3)
Nevertheless, we are asked to find this value in Joules. So, we have to convert this 32625 calories to Joules, knowing the following:
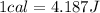 (4)
(4)
Hence: