Answer: The pair of valence electrons that do not participate in the bonding are lone pairs.
Step-by-step explanation:
There are 2 types of pair of electrons that are studied in the bonding. They are:
- Bonding pairs: These pair of electrons participate in the bond formation. They exist as bonds in a molecule. They are also known as bond pairs.
- Non-bonding pairs: These pair of electrons do not participate in the bond formation. They exist as lone pairs in a molecule.
Oxygen is the 8th element of the periodic table having electronic configuration of
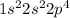
Number of valence electrons = (2 + 4) = 6
Oxygen needs 2 electrons to complete its octet. When another oxygen combines, they share 2 electrons each forming a double bond.
The Lewis dot structure of oxygen molecule is given below.
Hence, the pair of valence electrons that do not participate in the bonding are lone pairs.