Answer : The correct option is, (C) 70%
Explanation : Given,
Molar mass of Si = 28.09 g/mole
Molar mass of C = 12 g/mole
Molecular mass of SiC = 28.09 + 12 = 40.09 g/mole
To calculate the mass percent of an element in a compound, we use the equation:
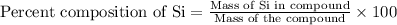
Now put all the given values in above equation, we get:
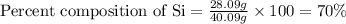
Hence, the percentage composition of Si in the given compound is 70%