Answer:
Concentration of the acid = 1.05M
Step-by-step explanation:
Given parameters:
Volume of acid = 14.0mL = 0.014L
Volume of base = 28.23mL = 0.02823L
Concentration of the base = 0.521M
Unkown:
Concentration of acid = ?
The reaction equation is given below:
CH₃COOH + NaOH → CH₃COONa + H₂O
To obtain the unknown concentration of the acid used in the reaction, we simply work from the known parameters of the given base.
Procedure:
- Find the number of moles of the base used in the reaction using the equation below:
Number of moles of base = concentration of base x volume
Number of moles of base = 0.521 x 0.02823 = 0.0147mol
- From the balanced reaction equation, we know that:
1mole of the base neutralize on mole of acid,
Therefore, 0.0147mol of the acid would be neutralized by the base.
To find the concentration of the acetic acid,
Concentration of acid =
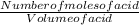
Concentration of acid =
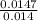
Concentration of the acid = 1.05M